Abstract
BACKGROUND: Maintenance rituximab in MCL has improved survival and supports the exploration of maintenance with novel targeted agents. Ibrutinib is a BTK inhibitor approved for relapsed/refractory MCL. We report the final analysis of safety and efficacy of Ibrutinib maintenance (I-M) as monotherapy following chemo-immunotherapy induction for treatment-naive MCL in a multicenter phase II trial.
METHODS: Pts with CR/PR to frontline chemo-immunotherapy (+/- autologous stem cell transplant (autoSCT)) received I-M 560 mg daily for up to 4 years. The primary endpoint was 3-year PFS rate. Secondary endpoints were to determine PR to CR conversions, median OS and the safety profile of I-M. Minimal residual disease (MRD) was measured using an NGS-MRD assay on peripheral blood (detection resolution of 1 cell per million; clonoSEQ®; Adaptive Biotechnologies) prior to and 1, 6 and 18-24 mo(s) after initiation of I-M.
RESULTS: 36 pts were enrolled to complete accrual. Median age was 60 years (range 46-90). For induction, most pts were treated with BR (n=17, 47%) or a cytarabine-containing regimen (n=18, 50%). Eighteen (50%) pts underwent autoSCT. Thirty-four (94%) and 2 (6%) had CR and PR as best response to induction respectively, with 1 PR to CR conversion on I-M.
At a median follow-up of 47 months, 10 (28%) pts completed a full I-M course, 7 (19%) remain on I-M, 15 (42%) discontinued I-M for treatment related adverse events (TRAEs) and 4 (11%) discontinued I-M for other reasons (PD x 1, secondary malignancies requiring treatment x 2, death cause unknown x 1). Three pts died during I-M, 2 deaths deemed unrelated to I-M (aspiration pneumonia, 2 nd malignancy) and 1 from unknown cause; 1 pt was lost to follow-up. Four pts were treated with rituximab maintenance after stopping I-M prematurely for toxicity without evidence of disease progression prior to or after change in therapy.
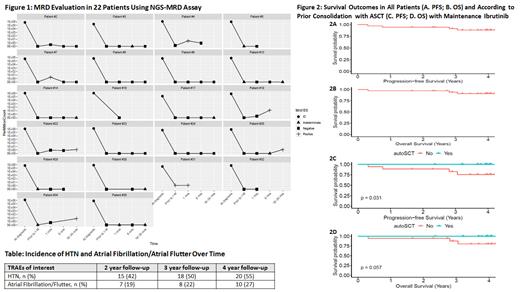
At the time of data cut-off, MRD was assessed in 22 of 36 pts (available samples) at varying time points (Fig 1) with a dominant clone identified in all 22 pts. Pts were deemed MRD (-) if no sequences were detected at a threshold of <10 -6 and (+) if sequences were detected at >10 -6. Seventeen pts were MRD (-), 4 MRD indeterminate and 1 MRD (+) with radiographic CR after induction; the latter remained MRD (+) at 18 months with CR. All MRD indeterminate pts were MRD (-) when checked after 1 month on I-M. Six pts MRD (-) post-induction became MRD (+) during their I-M course. Of these pts, 2 reverted to MRD (-) with continued I-M; of the remaining 4 pts, 1 had PD and the others maintain stable clinical responses with ongoing I-M though MRD has not been rechecked.
3-year PFS and OS rates were 91% and 94% respectively (Figure 2A, B). PFS was improved in pts who received autoSCT prior to enrollment (Fig 2C, p=0.03) with a trend for improved OS (Figure 2D, p=0.057). MRD did not correlate with PFS (p=0.65) and OS (p=0.45) given few events.
Atrial fibrillation/flutter occurred in 10 pts (28%; G1-2 n=7, 19%, G≥3 n = 3, 8%), 8 (22%) with new onset and 2 (6%) with worsening grade. HTN occurred in 20 pts (55%; G1-2 n=13, 33%, G≥3 n = 7, 22%), 15 (42%) with new onset and 5 (14%) with worsening grade. Incidences of both atrial fibrillation/flutter and HTN increased over time with ongoing I-M exposure (Table). Four pts had a 2 nd solid malignancy, 2 while on treatment and 2 after stopping I-M. TRAEs led to permanent dose reductions in 8 (22%) pts, 2 for neutropenia, 2 for fatigue, 2 for myalgias, 1 each for diarrhea and mucositis. Fifteen (42%) pts permanently discontinued I-M, most commonly for atrial fibrillation/flutter (n=8, 22%; n=5, 14% for G1-2).
CONCLUSION: I-M 560 mg daily after response to frontline chemo-immunotherapy is feasible in MCL and results in durable PFS and OS. Toxicities including rates of high-grade atrial fibrillation/flutter and HTN are consistent with ibrutinib's known safety profile with increased incidence with longer exposure; discontinuation of I-M for atrial fibrillation/flutter in 22% of pts is higher than expected. Changes in NGS-MRD were noted in a small number of pts during maintenance. Extended follow-up and correlation of changes in MRD with PFS and OS are needed to determine clinical relevance of I-M and MRD status. Further studies evaluating maintenance with next generation BTK inhibitors as alternatives to ibrutinib should be explored to mitigate toxicity.
Karmali: Takeda: Research Funding; Genentech: Consultancy; Roche: Consultancy; Epizyme: Consultancy; Morphosys: Consultancy, Speakers Bureau; BeiGene: Consultancy, Speakers Bureau; Janssen/Pharmacyclics: Consultancy; BMS/Celgene/Juno: Consultancy, Research Funding; AstraZeneca: Speakers Bureau; Karyopharm: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding, Speakers Bureau; EUSA: Consultancy. Abramson: Seagen Inc.: Research Funding; Allogene Therapeutics: Consultancy; Astra-Zeneca: Consultancy; Incyte Corporation: Consultancy; BeiGene: Consultancy; Kymera: Consultancy; Bluebird Bio: Consultancy; Genmab: Consultancy; EMD Serono: Consultancy; Bristol-Myers Squibb Company: Consultancy, Research Funding; Novartis: Consultancy; Kite Pharma: Consultancy; Morphosys: Consultancy; C4 Therapeutics: Consultancy; AbbVie: Consultancy; Karyopharm: Consultancy; Genentech: Consultancy. Stephens: JUNO: Research Funding; Abbvie: Consultancy; Adaptive: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Innate Pharma: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; AstraZeneca: Consultancy; CSL Behring: Consultancy; Celgene: Consultancy; Mingsight: Research Funding; Arqule: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Epizyme: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding. Winter: Ariad/Takeda: Other: Husband: Data and Safety Monitoring Board; Actinium Pharma: Consultancy; BMS: Other: Husband: Data and Safety Monitoring Board; Gilead: Other: Husband: Consultancy; Janssen: Other: Husband: Consultancy; Epizyme: Other: Husband: Data and Safety Monitoring Board; Agios: Other: Husband: Consultancy; Novartis: Other: Husband: Consultancy, Data and Safety Monitoring Board; Merck: Consultancy, Honoraria, Research Funding; Karyopharm (Curio Science): Honoraria. Ma: Juno: Research Funding; Beigene: Research Funding, Speakers Bureau; AstraZeneca: Honoraria, Research Funding, Speakers Bureau; Loxo: Research Funding; Janssen: Research Funding, Speakers Bureau; Abbvie: Honoraria, Research Funding; TG Therapeutics: Research Funding; Pharmacyclics: Research Funding, Speakers Bureau. Petrich: Daiichi-Sankyo: Current Employment; Abbvie: Ended employment in the past 24 months. Hochberg: Leuko: Consultancy; Trapelo Health: Consultancy. Kuhr: Adaptive Biotechnologies: Current Employment. Lee: Adaptive Biotechnologies: Current Employment. Gordon: Zylem Biosciences: Patents & Royalties: Patents, No royalties; Bristol Myers Squibb: Honoraria, Research Funding.
We will report on the use of ibrutinib maintenance after front-line induction therapy in MCL.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal